FDA gives Cue Health first EUA for at-home, OTC COVID-19 molecular testing

Russian, Chinese hackers may have stolen European vaccine data
08/03/2021
COVID-19 : le vaccin est réellement disponible sur le Dark Web
11/03/2021 
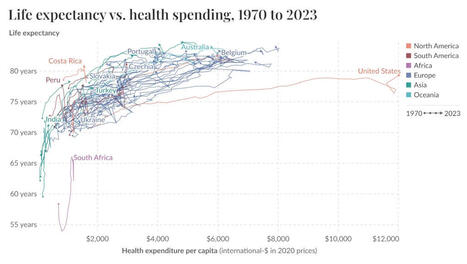
The FDA has issued its first emergency use authorization for a COVID-19 molecular diagnostic test that does not require a prescription and can be used at home.
Developed by Cue Health, the Cue COVID-19 Test for Home and Over the Counter Use was first authorized for point-of-care use last June.
It uses a nasal swab sample collected from the lower nose to detect the presence of SARS-CoV-2 RNA, and transmits its results directly to the corresponding Cue Health App. The test includes a single-use Cue Sample Wand and a single-use Cue Test Cartridge – but also requires the Cue Cartridge Reader, a separate device that is reusable and battery operated.
The molecular nucleic acid amplification test generates and transfers its results to the app within “about 20 minutes,” according to the agency. Of note, consumers using the system will need to first create an account. Further down the line, the agency said that the app will eventually be able to report those results to public health authorities for wider-scale disease monitoring.
Lire l’article complet sur : www.mobihealthnews.com