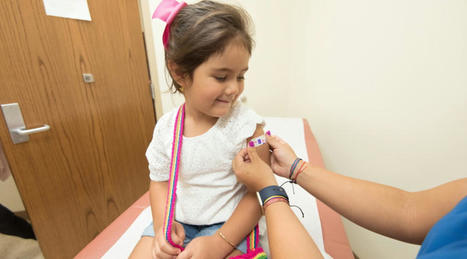EU authorises first COVID-19 vaccine for 5 to 11 year olds

The WHO let Omicron skip over variant of interest, and go straight to variant of concern
28/11/2021
Changes in vital data after a COVID-19 infection ·
28/11/2021 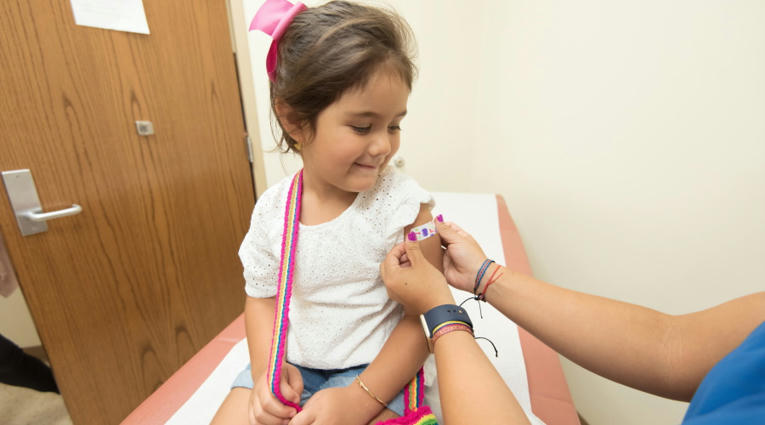
The European Medicines Agency (EMA) has approved Pfizer and BioNTech’s COVID-19 vaccine Comirnaty for children between the ages of five and 11, as EU countries struggle to cope with rising infection rates.
The EU regulator said that the under-12s should get a lower dose of the vaccine – 10 mcg rather than 30 mcg – given as two intramuscular injections three weeks part. The US approved Comirnaty for this age group last month.
So far the UK’s Medicines and Healthcare products Regulatory Agency (MHRA) hasn’t delivered its verdict on the use of Comirnaty in younger children.
However, it has been reported that the UK government is already planning to extend its vaccination programme to the five to 11 age group next year – subject to approval from the Joint Committee on Vaccines and Immunisation (JCVI).
Lire l’article complet sur : pharmaphorum.com