Pharmageek

Grâce aux technologies de pointe et aux innovations d'aujourd'hui, nous avons accès aux soins de santé les plus avancés. En effet, l’événement qu’il ne fallait pas rater fin octobre était l'Epicenter Health & MedTech Innovation Days, en Suède. Epicenter est la première Maison de
Lire l'article complet sur : iatranshumanisme.com

PARIS (TICsanté) - Les députés ont approuvé le 26 novembre en première lecture, à l'unanimité, une proposition de loi tendant à mettre sur pied une plateforme de référencement et de prise en charge des malades chroniques du Covid-19.
Lire l'article complet sur : www.ticsante.com
Demographie In den bisherigen Blogposts haben wir die räumliche Verteilung der Spender:innen und die gespendeten Schrittzahlen und Pulsraten beleuchtet. In diesem Blogpost beschäftigen wir uns nun eingehender mit der Demographie der Spender:innen. Hierzu gehören die Informationen zu Alter, Geschlecht, Größe, und Gewicht, die Nutzer:innen in der App angeben können.
Einige Spender:innen (etwa 10%) haben neben ihren täglichen Ruhepuls- und Schrittanzahldaten auch freiwillig Informationen zu Alter, Geschlecht, Größe und Gewicht angegeben.
Lire l'article complet sur : corona-datenspende.de
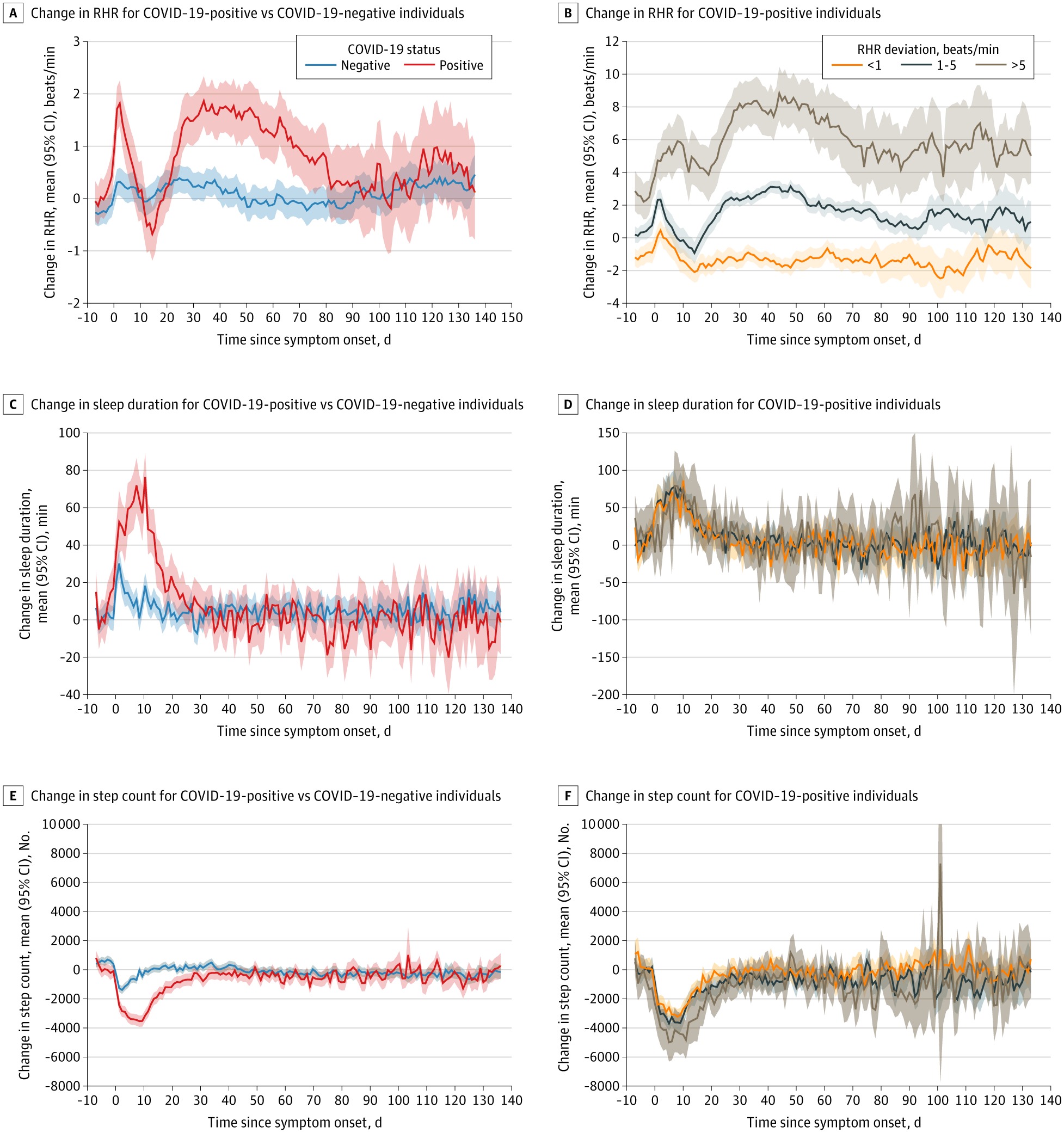
This cohort study examines the duration and variation of recovery among COVID-19–positive verses COVID-19–negative individuals.
Lire l'article complet sur : jamanetwork.com