Scoop.it
22/04/2020
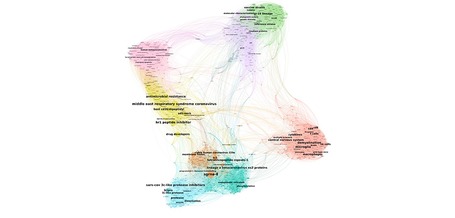
L’Institut des systèmes complexes de Paris Île-de-France a publié plusieurs cartes construites à partir de l’analyse automatisée de toutes les publications scientifiques consacrées au Covid-19. Son directeur David Chavalarias nous explique l'intérêt de ces visualisations pour la recherche, et pour le public.
Source: lejournal.cnrs.fr
22/04/2020
COVID-19 is proving to be a disease of the immune system. This could, in theory, be controlled.
Source: www.theatlantic.com
22/04/2020
Téléconsultation, vidéoconférence, téléexpertise, télésuivi des patients, tableau de bord, les réponses numériques face à la crise sanitaire due à l’épidémie de COVID-19 constituent un levier majeur pour limiter les contacts physiques, fluidifier la concertation et surtout optimiser la...
Source: www.dsih.fr
22/04/2020
Thanks to an updated emergency use authorization (EUA) from the FDA, LabCorp's COVID-19 RT-PCR test is now the first diagnostic test for COVID-19 that permits at-home sample collection.
Now, patients may use Q-tip-style nasal cotton swabs and saline included in LabCorp's designated self-collection kit – which the company is branding as the "Pixel by LabCorp COVID-19 Test." These samples are mailed to a LabCorp facility in an insulated package for molecular testing.
LabCorp said in an announcement that it will initially be releasing these kits to healthcare workers and first responders with symptoms of COVID-19 or potential exposure to the virus. For consumers, the self-collection kits will be available in "most states" in the coming weeks, and will require a doctor's order, according to the FDA.
"For tests that include home sample collection, we worked with LabCorp to ensure the data demonstrated from at-home patient sample collection is as safe and accurate as sample collection at a doctor’s office, hospital or other testing site," FDA Commissioner Dr. Stephen M. Hahn said in a statement. "With this action, there is now a convenient and reliable option for patient sample collection from the comfort and safety of their home.”
Source: www.mobihealthnews.com