Scoop.it
26/04/2020
Recent moves by the Food and Drug Administration have given digital health companies a big boost during the Covid-19 pandemic. The FDA gave Livongo an emergency use authorization to allow its blood glucose monitors to be used in hospitals, and has loosened requirements around the use of digital tools for mental health.
Source: medcitynews.com
26/04/2020
Covid-19 and Health Care’s Digital Revolution In the face of the Covid-19 pandemic, Americans are waking up to the limitations of their analogue health care system. It seems clear that we need a
Source: www.nejm.org
26/04/2020
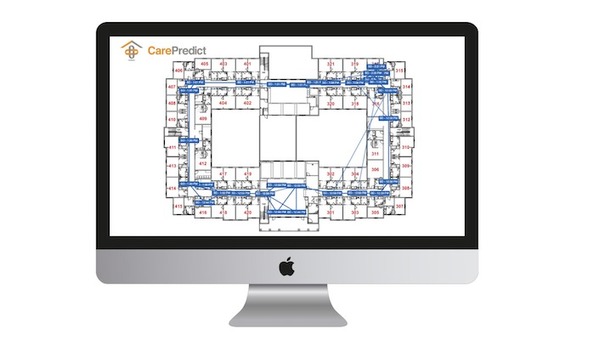
The tool is able to track who a patient or staff member came into contact with and where they traveled in the facility.
Source: www.mobihealthnews.com
26/04/2020
Mobile and tablet apps, ubiquitous and pervasive computing, wearable computing and domotics for health.
Source: mhealth.jmir.org