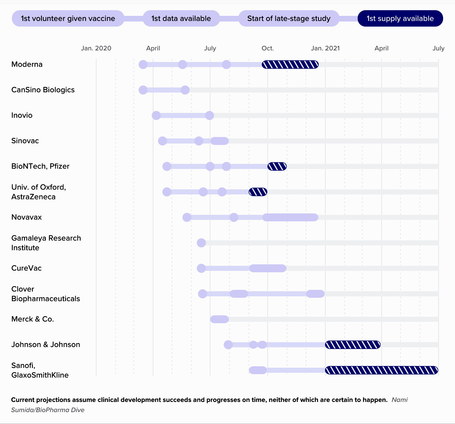
Scientists, drugmakers and governments are advancing coronavirus vaccines quickly. Here is the current status:

SimforHealth déploie son logiciel de formation au diagnostic du Covid-19 bravo @jeromeleleu
21/08/2020
Coronavirus. Pourquoi les experts « vus à la télé » ne s’accordent-ils pas sur un même message ?
21/08/2020Scientists, drugmakers and governments are moving with unprecedented haste to deliver a vaccine to protect against the new coronavirus. The fastest of them have already delivered early data from human studies, and further results from others should come quickly as the year progresses.
The goal, at least in the U.S., is to have a vaccine ready for use in some fashion by the end of the year, or early next. Doing so would be a scientific feat with few parallels. No vaccine has ever been developed so quickly, never mind manufactured for the world.
Current projections assume clinical development succeeds and progresses on time, neither of which are certain to happen. Researchers’ success or failure could determine whether the virus becomes endemic, recurring in countries around the world year after year, or is ultimately checked.
With the health of their citizens at stake, governments are investing enormous sums of money into vaccine research and development, and to prepare for manufacturing and distributing what will likely need to be hundreds of millions of doses necessary to keep infection at bay.
With modern-day Manhattan Projects underway, vaccines have become an issue of national security, too, raising questions of global equity and medicine access.
In the U.S., the Trump administration has unveiled "Operation Warp Speed," so far pledging more than $11 billion in funding and support for seven candidates.
There’s no guarantee the first successful vaccine will come from the U.S., however. Some of the leading candidates are being developed overseas, with projects by the University of Oxford in the U.K. and China’s CanSino Biologics the furthest along.
Source: www.biopharmadive.com